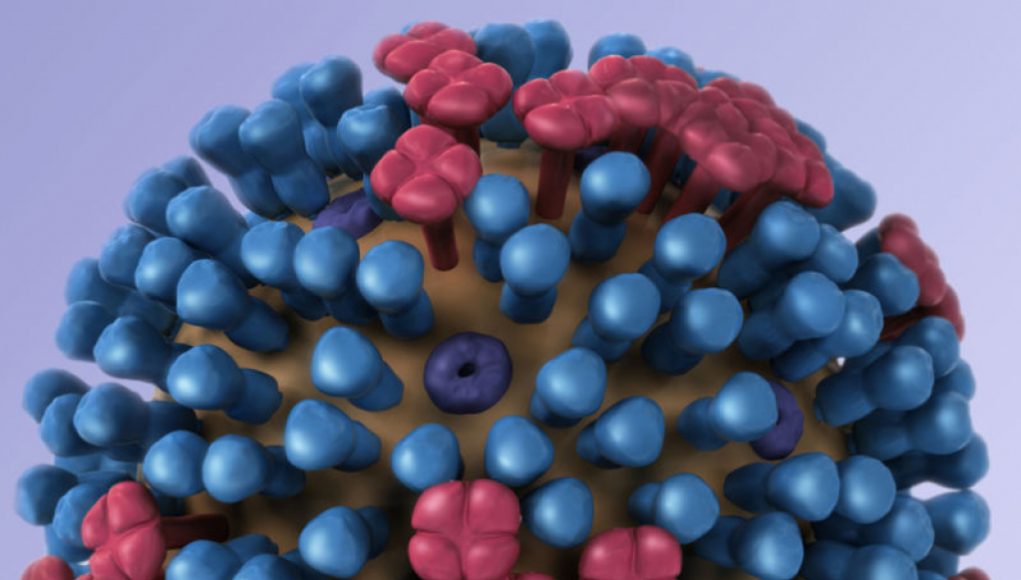
The National Institutes of Health has announced an exciting new development in the fight against influenza. A phase I clinical trial is underway for an mRNA-based flu vaccine that could offer long-lasting protection against a broad range of influenza viruses. This is a major breakthrough in the quest for a universal flu vaccine, which would eliminate the need for annual reformulation of flu shots to match circulating strains. The mRNA vaccine platform targets a highly conserved part of the Ha protein’s stem, which doesn’t evolve as quickly as other parts of the protein. This means that the vaccine could induce long-term immunity. The trial is starting with a small group of 50 people, but the researchers are hopeful that having multiple platforms in the works will increase their chances of success. A universal flu vaccine would be a major public health achievement and could serve as an important line of defense against the spread of a future flu pandemic.
The world of medical science just took a major step forward with the start of a clinical trial for a Universal Flu Vaccine (UFF). This is a massive milestone for the development of this revolutionary vaccine and marks an important chapter in efforts to control influenza-related illnesses and deaths around the world.
UFF is made from messenger RNA (mRNA), a genetic molecule that carries genetic information from the nucleus of a cell to its protein-making structures. Developed at the mRNRS, a biotech firm in Harvard University’s Wyss Institute, the mRNA-based vaccine is designed to protect against all strains of the flu virus.
The clinical trial will involve 180 healthy adults who will receive either a single dose of the UFF or a placebo. The volunteers, who will be monitored over a period of one year, will be tested for safety and exemptions to latent autoimmune disorders, while samples of their blood will be taken to assess how effectively the vaccine is producing immunity against the flu virus.
The benefits of a vaccine as effective and long-lasting as UFF could be enormous. By protecting against all strains of the flu virus, it could spare millions of people from the dangerous diseases that can result from the Influenza A and B viruses. Moreover, it would make it unnecessary to invest in annual influenza vaccinations that are time-consuming for patients and health care providers and can lose their effectiveness if mismatched to the strains of the virus circulating each season.
While this trial is only a starting point, the accomplishment of bringing the technology this far is substantial, and the hope is that it will be able to successfully complete the three phases of clinical trials required for approval by the Food and Drug Administration.
Overall, the launch of this trial is a milestone in the development of a genuinely groundbreaking and life-saving vaccine. It is a major breakthrough and a new chapter in medical science, and potentially a major step towards dramatically reducing the burden of influenza-related diseases.