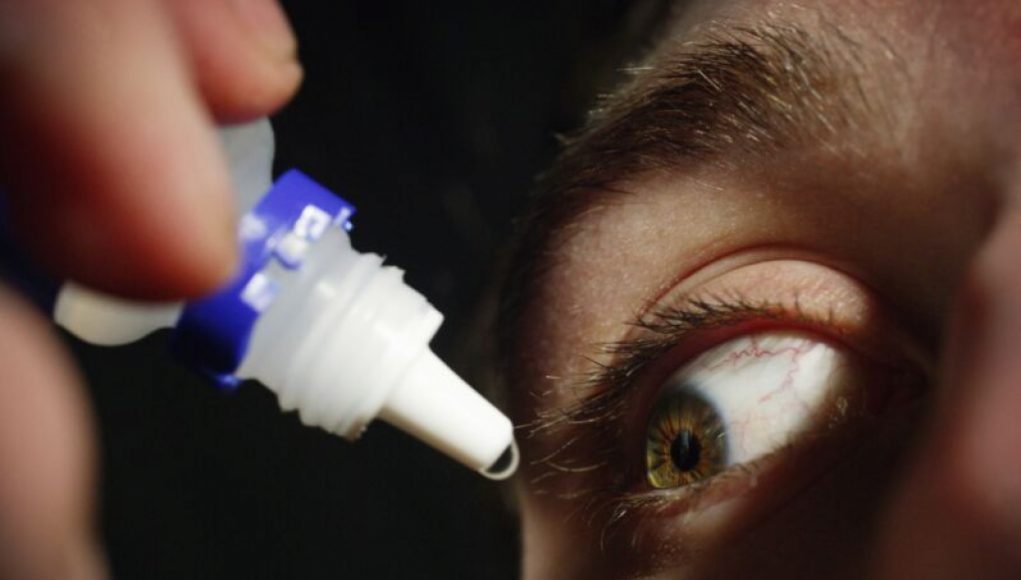
In a shocking update, the Centers for Disease Control and Prevention have reported that another person has died in an outbreak of extensively drug-resistant bacteria linked to contaminated eye drops. The outbreak has now spread to 81 cases across 18 states, with reports of 14 people suffering from vision loss and four people having their eyeballs surgically removed due to infection. The bacteria behind the outbreak is a strain of Pseudomonas aeruginosa dubbed VIM-GES-CRPA, an extensively drug-resistant strain of bacteria that had never been seen in the US before. The bacteria’s origins have been traced to contaminated eye drops, with EzriCare artificial tears being the most common product used by people infected during the outbreak. The FDA has advised people to stop using these products immediately if they haven’t already.
This week’s outbreak update includes 13 new cases since the last outbreak update in March, six of which had samples collected prior to the recall and are now confirmed and added to the tally. Of the seven other newly added cases, most either resided in long-term care facilities with other known cases or reported continued use of one of the recalled artificial tears, the CDC reported.
The outbreak strain is particularly concerning because it can spread quietly from person to person and share drug-resistance genes with other pathogens. The widespread introduction of the outbreak strain threatens to undermine efforts to prevent these highly resistant organisms from becoming more common. The CDC has advised doctors to work with specialists to determine treatment plans for the extensively drug-resistant pathogen.
Researchers at the University of California at San Diego’s Center for Innovative Phage Applications and Therapeutics (IPATH) and the Yale Center for Phage Biology and Therapy have identified bacteriophage with activity against the outbreak strain. Bacteriophage are viruses that selectively infect and destroy bacteria, and researchers have for decades explored ways to use them to treat bacterial infections.
While doctors and researchers work to treat the tenacious infections, some patients have already filed lawsuits against EzriCare, Global Pharma, and retailers. The FDA posted an inspection report of Global Parma’s facility, finding a slew of manufacturing violations, slime on equipment, and a lack of measures to ensure sterility. Stay safe and stay informed.
An outbreak of an eye infection spread by contaminated eye drops has caused alarm in recent weeks, as it has been reported to have affected 18 states in the United States and has claimed the life of one individual.
The infection, caused by a bacteria, has been linked to a single brand of eye drops, Altaire Pharmaceuticals’ Advanced Eye Relief Drops 0.5% tetracaine hydrochloride. The CDC has been investigating since August and released a statement on October 11th recommending individuals “not to use any ophthalmic products made by Altaire Pharmaceuticals, Inc.”
So far, the outbreak has caused 37 cases of Acanthamoeba keratitis, an eye infection that is usually caused by contact with the Acanthamoeba parasite in contaminated water or soil. Symptoms include blurred vision, redness and pain in the eyes, and sensitivity to light. If left untreated, the infection can lead to permanent scarring of the surface of the cornea, partial or complete blindness, and even death.
According to the CDC, the outbreak is likely to continue to spread, especially in the Midwest and Southeastern regions of the United States where temperatures remain hot and humid. The agency also states it “may be difficult for the public to determine which products are made by Altaire Pharmaceuticals, Inc. and may have been contaminated.”
The makers of Altaire Pharmaceuticals, Inc. have recalled the implicated eye drops, but it may be too late for those already affected. The CDC is warning anyone with inflamed eyes to seek medical attention in order to receive treatment as soon as possible.
It is unclear yet what caused the eye drops to become contaminated with the bacteria, however, it serves as a reminder to the public to always be vigilant when it comes to the safety of products and medical treatments.