Did you know that scientists have now taken the first X-ray of a single atom? That’s right! Researchers from Ohio University, Argonne National Laboratory, and the University of Illinois-Chicago have achieved this incredible feat, as reported in the journal Nature. This breakthrough will have a significant impact on environmental and medical sciences, as it allows us to detect the type of atom and measure its chemical state, ultimately tracing materials down to just one atom.
Atomic-scale imaging has come a long way since the mid-1950s, when it first emerged. In 2008, physicists used an electron microscope to image a single hydrogen atom, and five years later, they were able to peer inside a hydrogen atom using a “quantum microscope,” resulting in the first direct observation of electron orbitals. Now, with the first X-ray taken of a single atom, the possibilities are endless.
When we think of an atom, we often picture the classic Bohr model, where electrons move around the atomic nucleus in circular orbits. However, this model has been superseded by the quantum world’s understanding, and Erwin Schroedinger proposed a new atomic model that dispensed with orbits in favor of energy levels. While electrons don’t technically “move” around the nucleus in orbits, they show up as particles when you perform an experiment to determine position. The electron doesn’t have a fixed position until you look at it, and the wave function collapses.
Advertisement
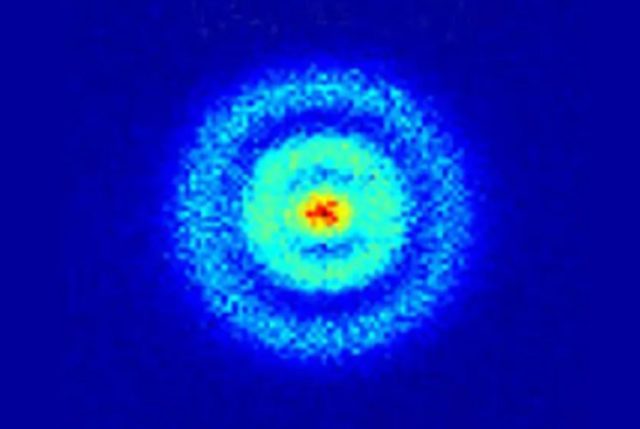
Despite the technicalities, this breakthrough in atomic-scale imaging is a significant step forward in our understanding of the world around us. Who knows what other incredible discoveries await us in the future?
Scientists from Lund University in Sweden have made an incredible breakthrough in the understanding of atomic-scale behaviour by capturing the first-ever X-ray image of a single atom.
This remarkable development was made possible by the use of an incredibly powerful microscope – the free-electron X-ray laser ‘SwissFEL’. The intense X-rays created by SwissFEL allowed the team to obtain a clear picture of the two electrons orbiting a magnesium nucleus.
The experiment involved firing a beam of magnesium ions at a gold target, which then evaporated, leaving single ions in their wake. The researchers focused a ‘tunneling electron microscope’ at the ions, which then recorded the electron density of the magnesium ion at a resolution of 3 picometers (3 x 10e-12m).
The capture of an X-ray image of a single atom could lead to a new understanding of atomic-scale behaviour and has the potential to revolutionise material science. In particular, this development could provide insight into the behaviour of matter in extreme environments, such as in space and near nuclear weapons. Furthermore, the team are hopeful that further experiments with other elements could yield further information on structure and behaviour at the atomic level.
Dr Børge Rosenbrand – one of the team members – commented on the achievement:
“It is remarkable what can be done today using X-ray laser technology. We are now looking into the details of how the electrons in the magnesium ion are arranged. This structure carries the unique signature of the magnesium ion, which allows us to distinguish them from other elements”.
This truly ground-breaking achievement has offered us an entirely new way of understanding atomic-scale behaviour, and the potential applications and discoveries this technique can bring are hugely exciting.